- Chromosomal microarray analysis (CMA) is a diagnostic test that can detect clinically significant large (whole chromosome) and submicroscopic (microdeletion/microduplication) copy number changes throughout the genome. Chromosomal microarray analysis is the gold standard for the detection of large deletions and duplications along the whole genome.The higher the resolution and the K value of the test, the higher the sensitivity.
WES Couple Overview
WES Diagnostic
- Whole Exome Sequencing can be used as a diagnostic tool for patients with complex genetic disorders, where the correct diagnosis is difficult to establish due to overlapping symptoms, complicated medical histories or in cases where previous genetic testing has not yielded conclusive results.
- Affected child with Normal karyotype, genetic condition suspected, undiagnosed condition and the Whole Exome Sequencing (WES) is Negative .
- Undiagnosed intellectual/developmental delay that does not fit a specific syndrome or Fragile-X syndrome.
- We can offer CMA to the parents in order to check whether the detected CNVs in the affectedchild is inherited or de novo.
- Abnormal fetal ultrasound
- High-risk maternal serum screening/NIPT result indicating an increased risk of a chromosomally abnormal fetus
- Parental chromosome rearrangement, mosaicism or previous aneuploidy
- Previous livebirth/stillbirth with a chromosome abnormality.
- Fetal congenital abnormalities detected on ultrasound or magnetic resonance imaging (MRI) that indicate a significant risk for an unbalanced chromosome abnormality.
- Apparently balanced inherited rearrangements in a fetus with fetal congenital abnormalities.
- Apparently balanced de novo rearrangements identified by G-band analysis
- High risk pregnancies
- Abnormal clinical phenotype
- Multiple congenital abnormalities
- A malformed fetus or stillbirth of unknown aetiology
- Clinically significant abnormal growth
- Short stature, excessive growth, microcephaly, macrocephaly
- Primary or secondary amenorrhea or ovarian insufficiency
- Ambiguous genitalia
- Idiopathic mental retardation / developmental delay / autism / multiple congenital abnormalities
- Apparently balanced inherited or de novo rearrangements in a phenotypically abnormal individual.
- Microarray is a first-line test in the initial postnatal evaluation in the following clinical scenarios:
- Multiple anomalies not specific to a well-delineated genetic syndrome
- Apparently non-syndromic developmental delay/intellectual disability
- Autism spectrum disorders
In these cases it may be especially useful when other tests have failed to yield a diagnosis:
- Unexplained seizure disorder
- Growth delay
- Psychiatric illness
- Neuromuscular conditions
- Spontaneous abortion
- POC sample
This test will detect:
- Small chromosomal microdeletions and duplications
- Chromosome number abnormalities
- Unbalanced rearrangements
- Mosaicism of greater than 20-25% can be detected by CMA
- Excessive homozygosity
- Triploid
-
Two different types of CMA are available in Igenomix – CystoScan HD and CytoScan 750K. These two tests are similar but have different indications with CMA HD being the more sensitive test and CMA750K being the most cost effective.
CytoScan HD array
- Copy number probes (1.9 million) + SNP (750 K)
- Whole genome coverage
- Can detect regions of low heterozygosity, uniparental disomy (UPD), low level mosciasim and sample heterogeneity
- Highest probe density
CytoScan 750K
- Copy number probes only (550K) + SNP (200 K)
- Emphasis on clinically relevant regions
- Identification of regions of excessive homozygosity indicating UPD, may suggest consanguinity and determine candidate genes for further testing
Deletions smaller than 50 kb and duplications smaller than 400 kb may not be reviewed. Detected copy number variations (CNVs) are reported when found to have clear or suspected clinical relevance; CNVs devoid of relevant gene content or reported as common findings in the general population may not be reported. Regions of homozygosity are reported when a single LCSH is greater than 8-15 Mb (dependent upon chromosomal location and likelihood of imprinting disorder), or when the total autosomal LCSH proportion is greater than 3% (only autosomal LCSH greater than 3 Mb are considered for this estimate). Genomic linear positions are given relative to NCBI build 37 (hg19).
Test results are interpreted based on the recommendations and guidelines of International Standard of Cytogenomics Arrays (ISCA) as described below:
A positive result indicates that a copy number variant has been identified in association with the disease phenotype under study. This scenario will allow to provide genetic counselling or personal guidance regarding possible medical treatments, disease progression, reproductive-/prevention-strategies and potential implications for other family members.
A negative result indicates that no disease-causing copy number variation was identified in the test performed. This does not guarantee that the induvial will be healthy or free from other genetic disorders or medical conditions. Additionally, a negative result does not rule out a genetic cause of the disease nor does it eliminate the risk for future offspring. However, if a negative test result is obtained and the variant in question is known to be present in affected family members, this then rules out a diagnosis of that genetic disorder in the proband. A negative result may be explained by several causes, including limited genetic knowledge and limitations associated to the used methodology.
A finding of a variant of uncertain significance indicates that a copy number variation was detected, but it is currently unknown whether that CNV is associated with a genetic disorder or disease. A variant of uncertain significance is not the same as a positive result and does not clarify whether the proband is at an increased risk to develop a genetic disorder or disease. The change could be a normal genetic variant, or it could be disease-causing. Further analysis may be recommended, including testing both parents as well as other affected and unaffected family members. Sometimes, performing ancillary tests is necessary to prove the phenotype that the proband presents with. Detailed medical records or information from other family members also may be needed to help clarify the result.
Result interpretation is based on currently available information in the medical literature, research, and scientific databases. Because the literature, medical and scientific knowledge are constantly changing, new information that becomes available in the future may replace or add to the information that Igenomix used to interpret the results. Re-analysis of the results in previously issued reports considering new evidence is not routinely performed but is available upon request
Reporting & Results
-
Two different types of CMA are available in Igenomix – CystoScan HD and CytoScan 750K. These two tests are similar but have different indications with CMA HD being the more sensitive test and CMA750K being the most cost effective.
CytoScan HD array
- Copy number probes (1.9 million) + SNP (750 K)
- Whole genome coverage
- Can detect regions of low heterozygosity, uniparental disomy (UPD), low level mosciasim and sample heterogeneity
- Highest probe density
CytoScan 750K
- Copy number probes only (550K) + SNP (200 K)
- Emphasis on clinically relevant regions
- Identification of regions of excessive homozygosity indicating UPD, may suggest consanguinity and determine candidate genes for further testing
Deletions smaller than 50 kb and duplications smaller than 400 kb may not be reviewed. Detected copy number variations (CNVs) are reported when found to have clear or suspected clinical relevance; CNVs devoid of relevant gene content or reported as common findings in the general population may not be reported. Regions of homozygosity are reported when a single LCSH is greater than 8-15 Mb (dependent upon chromosomal location and likelihood of imprinting disorder), or when the total autosomal LCSH proportion is greater than 3% (only autosomal LCSH greater than 3 Mb are considered for this estimate). Genomic linear positions are given relative to NCBI build 37 (hg19).
Test results are interpreted based on the recommendations and guidelines of International Standard of Cytogenomics Arrays (ISCA) as described below:
A positive result indicates that a copy number variant has been identified in association with the disease phenotype under study. This scenario will allow to provide genetic counselling or personal guidance regarding possible medical treatments, disease progression, reproductive-/prevention-strategies and potential implications for other family members.
A negative result indicates that no disease-causing copy number variation was identified in the test performed. This does not guarantee that the induvial will be healthy or free from other genetic disorders or medical conditions. Additionally, a negative result does not rule out a genetic cause of the disease nor does it eliminate the risk for future offspring. However, if a negative test result is obtained and the variant in question is known to be present in affected family members, this then rules out a diagnosis of that genetic disorder in the proband. A negative result may be explained by several causes, including limited genetic knowledge and limitations associated to the used methodology.
A finding of a variant of uncertain significance indicates that a copy number variation was detected, but it is currently unknown whether that CNV is associated with a genetic disorder or disease. A variant of uncertain significance is not the same as a positive result and does not clarify whether the proband is at an increased risk to develop a genetic disorder or disease. The change could be a normal genetic variant, or it could be disease-causing. Further analysis may be recommended, including testing both parents as well as other affected and unaffected family members. Sometimes, performing ancillary tests is necessary to prove the phenotype that the proband presents with. Detailed medical records or information from other family members also may be needed to help clarify the result.
Result interpretation is based on currently available information in the medical literature, research, and scientific databases. Because the literature, medical and scientific knowledge are constantly changing, new information that becomes available in the future may replace or add to the information that Igenomix used to interpret the results. Re-analysis of the results in previously issued reports considering new evidence is not routinely performed but is available upon request
WES Sample Requirements
- For genetic testing through next generation sequencing, the following sample types are accepted. A thorough labelling of the tube with unique identifying information is suggested, incorrect labelling can lead to rejection of the sample.
- The minimum required information to identify and accept a sample is – Patient’s full name, Date of birth, Gender and Medical Record Number.
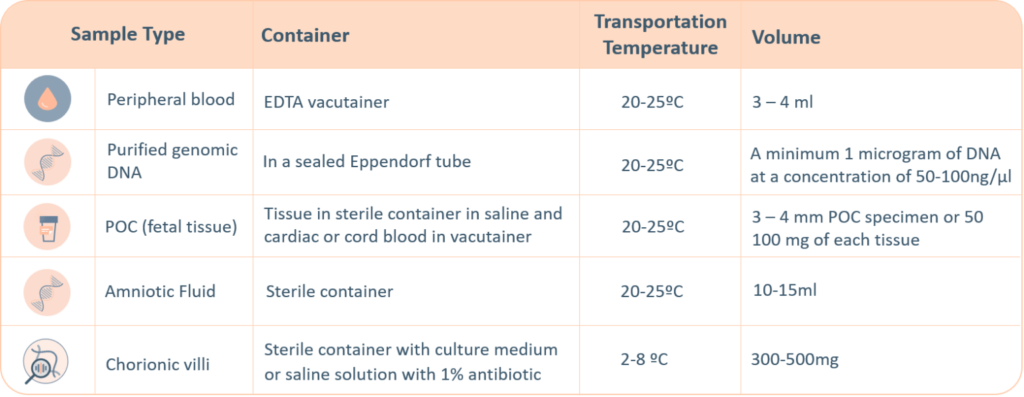
- Maternal blood sample must be sent with all products of conception, CVS and Amnio samples.
- Precedence will be given to all prenatal samples.
- The ‘informed consent’ form and the ‘test requisition from’ (included within the provided kit) must be properly filled-in and signed by the patient and sent with the samples inside the shipping box or by e-mail to the laboratory. Igenomix will send you all the documents needed for the pick-up and transportation of the appropriate kit to our laboratory
WES Couple Limitations
- Array-CGH analysis only identifies copy number variations viz., gain and losses along with regions of heterozygosity; does not allow the detection of balanced chromosome rearrangements, complete polyploidy (that is, for example, a 3n or a 4n karyotype) and aberrations present only in a certain number of cells (known as genetic mosaicism)
- CMA cannot identify:
- Balanced chromosomal rearrangements (balanced translocations, inversions)
- Small deletions (gene, exons, ..)
- Small changes in the sequence of single genes (point mutations)
- Tiny duplications and deletions of DNA segments within a single gene (Fragile X syndrome, for example)
- Uniparental disomy (UPD)
- Methylation alterations
- The limitations of CMA testing also vary with the methodology used. Most CMA cannot detect mosaicism below 20-25%. Some platforms do not detect excessive homozygosity or triploidy as well as others
For these reasons positive CGH microarray findings are usually followed up by FISH analysis. FISH can confirm if an array result is clinically significant and can also detect carriers of balanced chromosome abnormalities. FISH testing may need to be extended to the patient’s parents to refine the clinical interpretation of the microarray results.