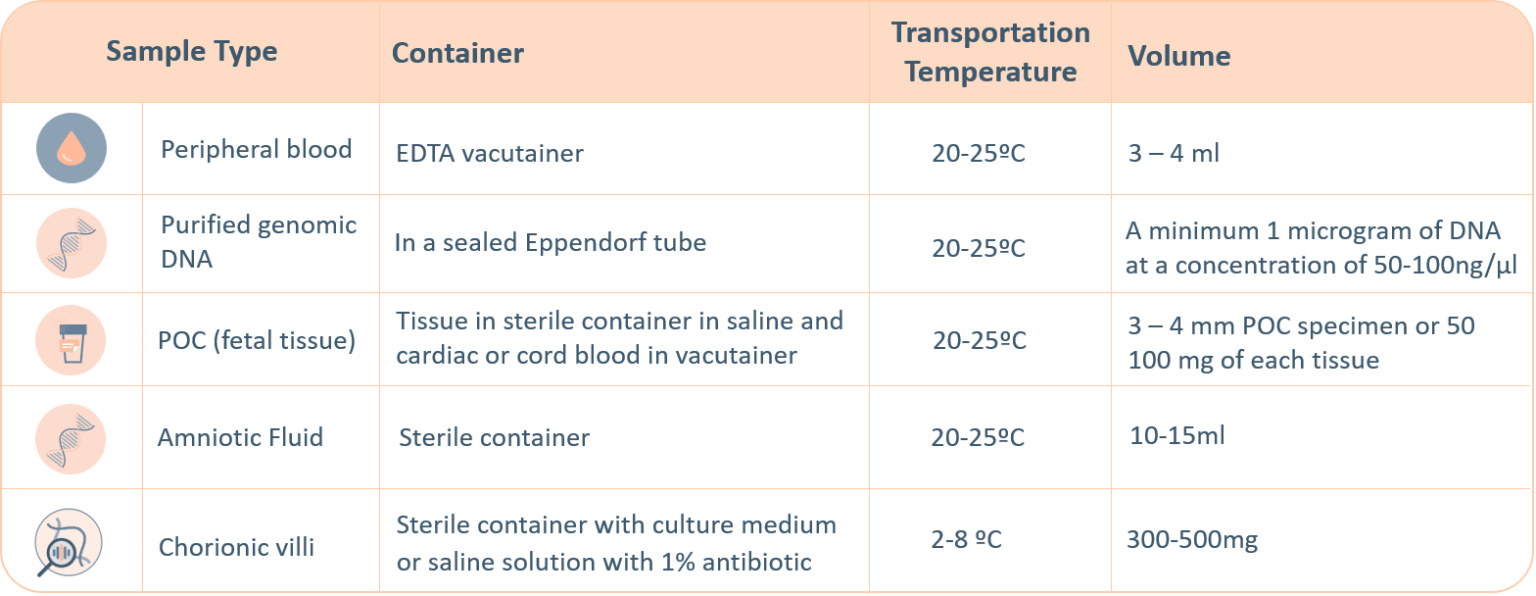
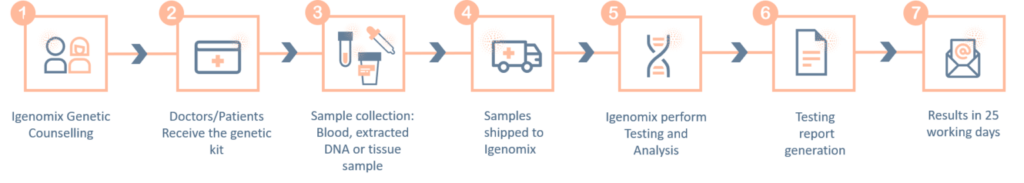
-
NGS Sequencing is a cost-effective technology for the diagnostic of mutations affecting to the entire coding region of a gen or small set of genes.
NGS – Next Generation Sequencing
Specific technology designed to detect DNA sequence variations in a gene or genomic region