Endocrine Cancer Precision Panel
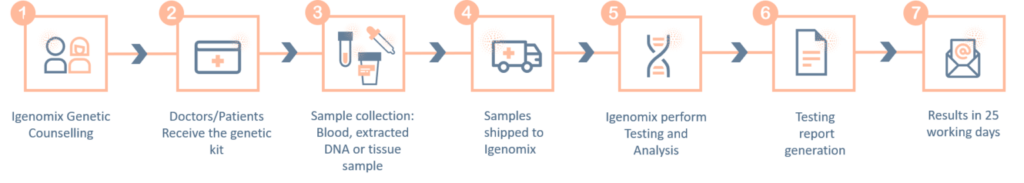
The Igenomix Endocrine Cancer Precision Panel has been designed to carry out an accurate and effective diagnosis of hereditary cancer syndromes involving the endocrine and neuroendocrine glands. It provides a comprehensive analysis of the most important genes associated with these types of cancer using next-generation sequencing.