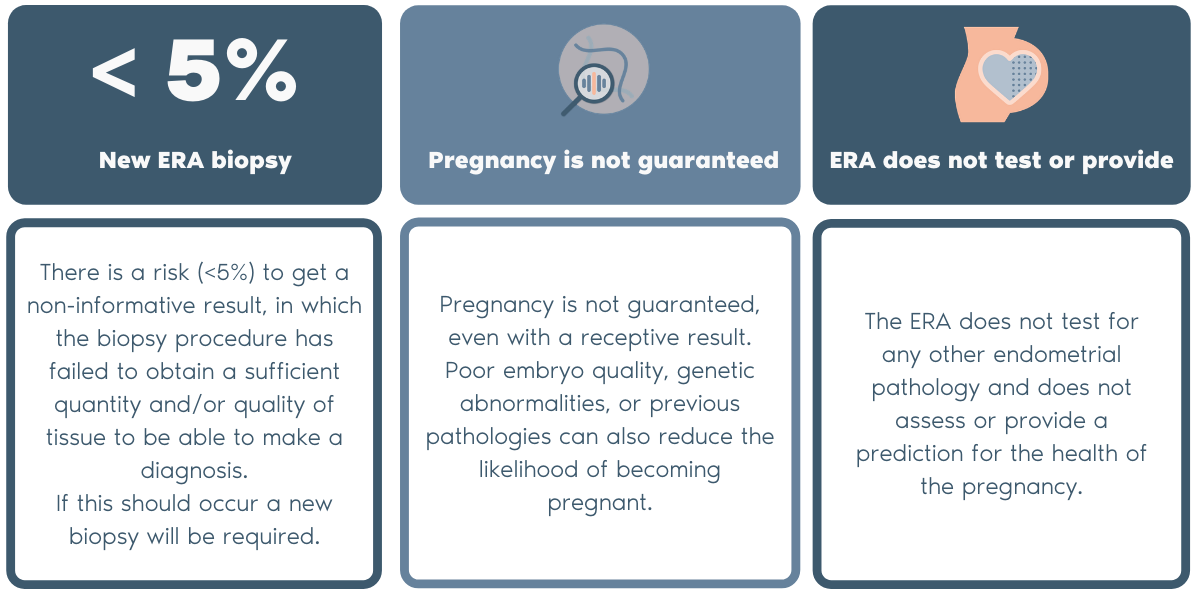
What is the ERA® (Endometrial Receptivity Analysis) test?
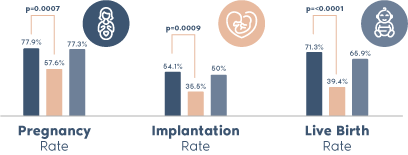
- ERA® test, developed and patented by Igenomix (PCT/ ES2009/000386), is a test designed to evaluate endometrial receptivity.
- ERA® is the first diagnostic test that determines each woman’s unique personalized embryo transfer timing, therefore synchronizing the embryo transfer with the individualized window of implantation.
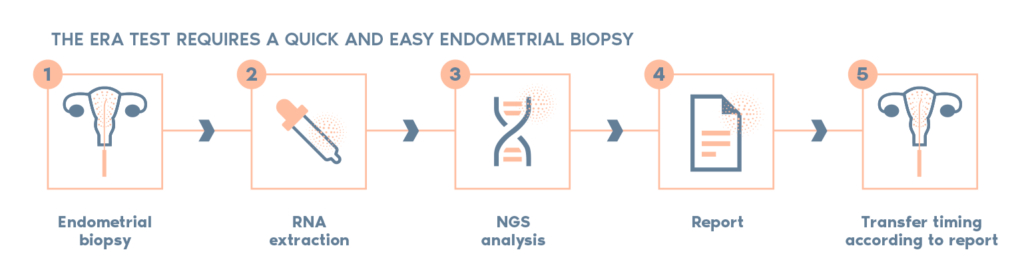
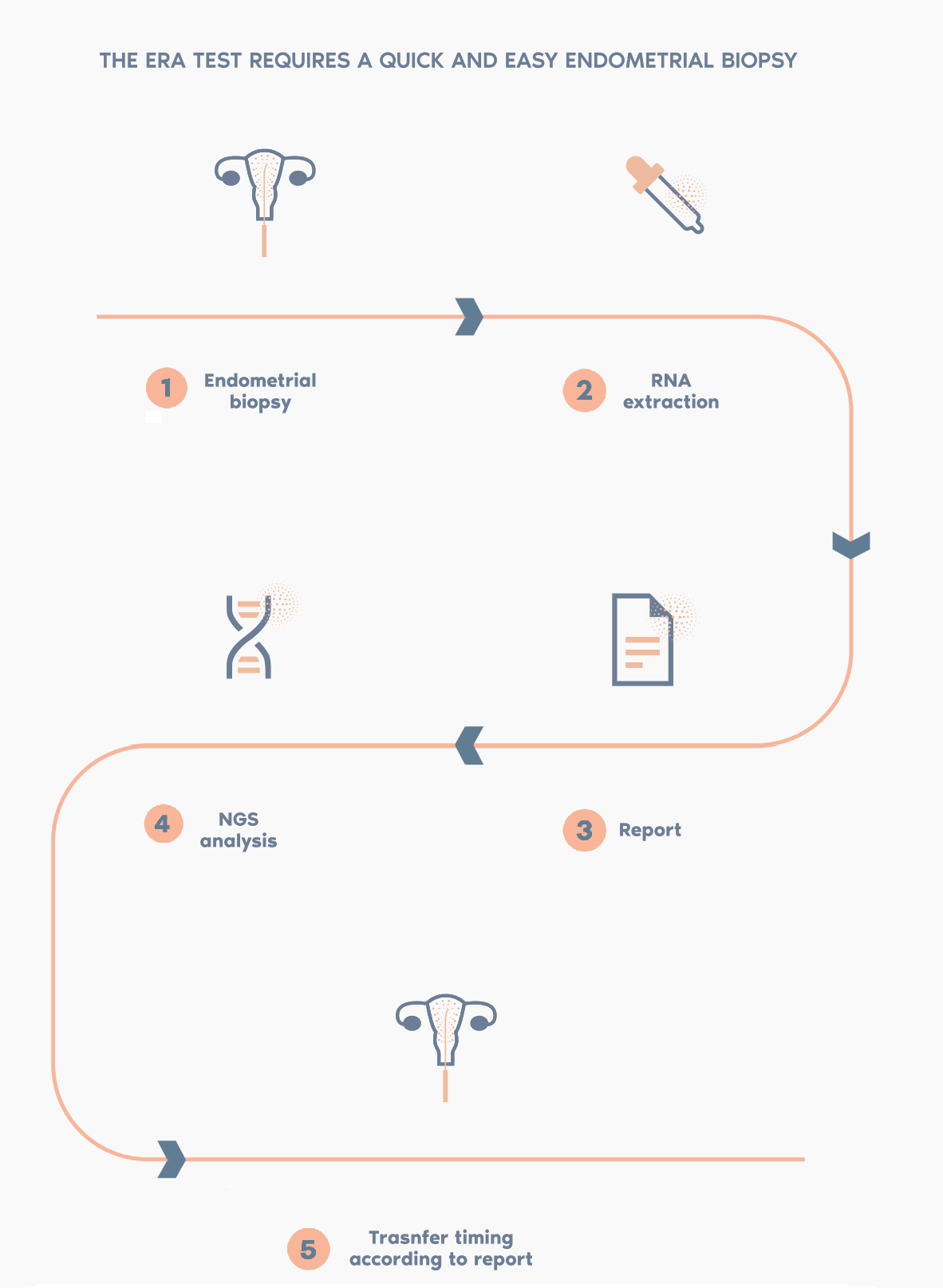
What is the procedure?