PGT-A Plus
PGT-A Plus
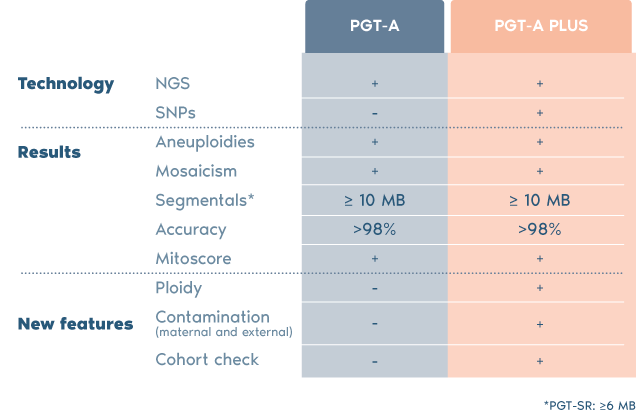
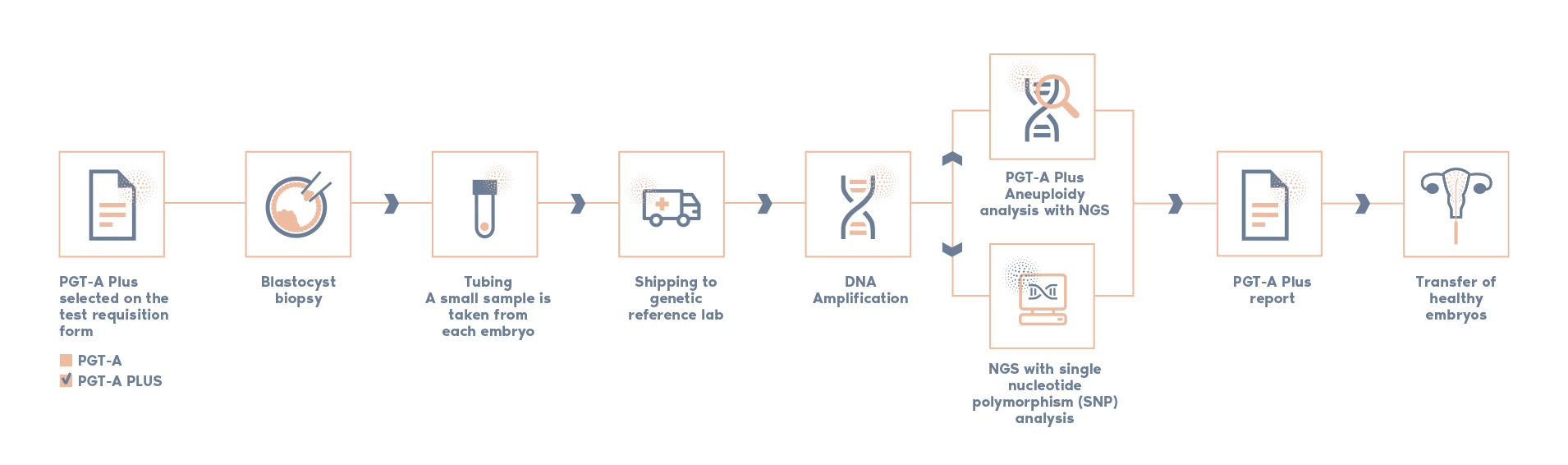
Our most advanced 4-in-1 genetic test offers increased accuracy and confidence for embryo transfer.

Combines two in-house validated technologies (NGS + SNPs analysis)
Superior accuracy and enhanced confidence to detect any abnormalities