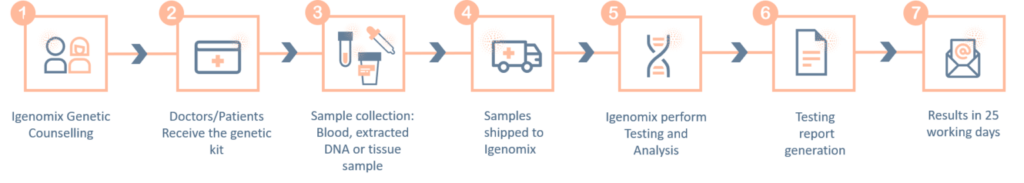
Oligohydramnios Precision Panel
Oligohydramnios is defined as an abnormally low volume of amniotic fluid. Amniotic fluid is crucial for fetal development and growth, serving as protection from trauma and infection and helping in the development of the fetal lungs.