What is the Oncodona Test?
It is the most advanced genetic test for early identification of predisposition to inherited cancer.
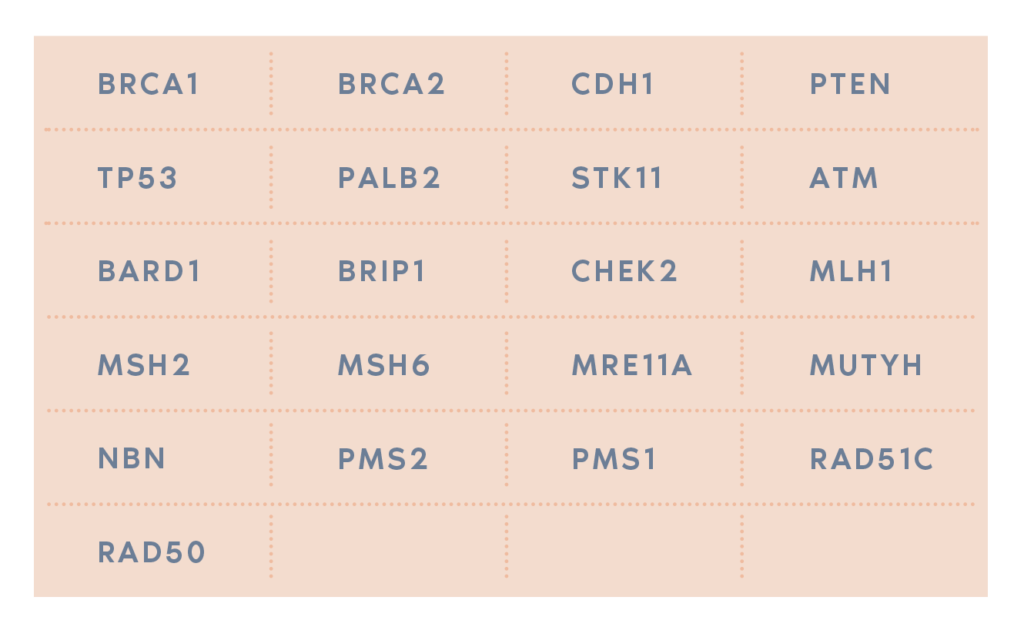
Oncodona uses Next Generation Sequencing (NGS) to analyze genetic information present in a panel of 21 genes, including BRCA1 and BRCA2 genes, in order to locate harmful mutations.
The presence of a change or mutation in one of these genes assumes that the female carrier has an increased risk of developing breast and ovarian tumours, or other related cancers.