What is POC?
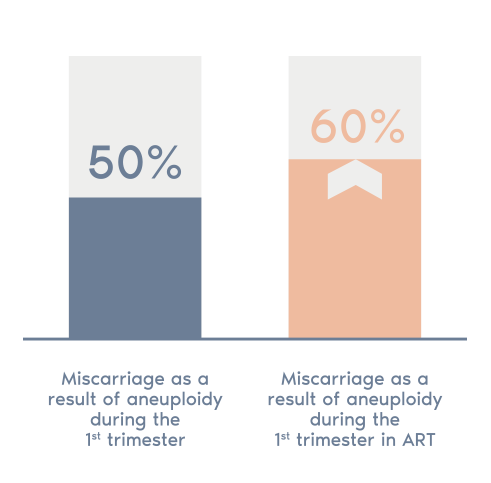
- The POC test analyzes fetal tissues from a miscarriage to determine if the lost pregnancy was the result of a chromosomal aneuploidy.
- This test can provide you an important information about the possible causes of your patient’s miscarriage to help them to plan any future pregnancy.
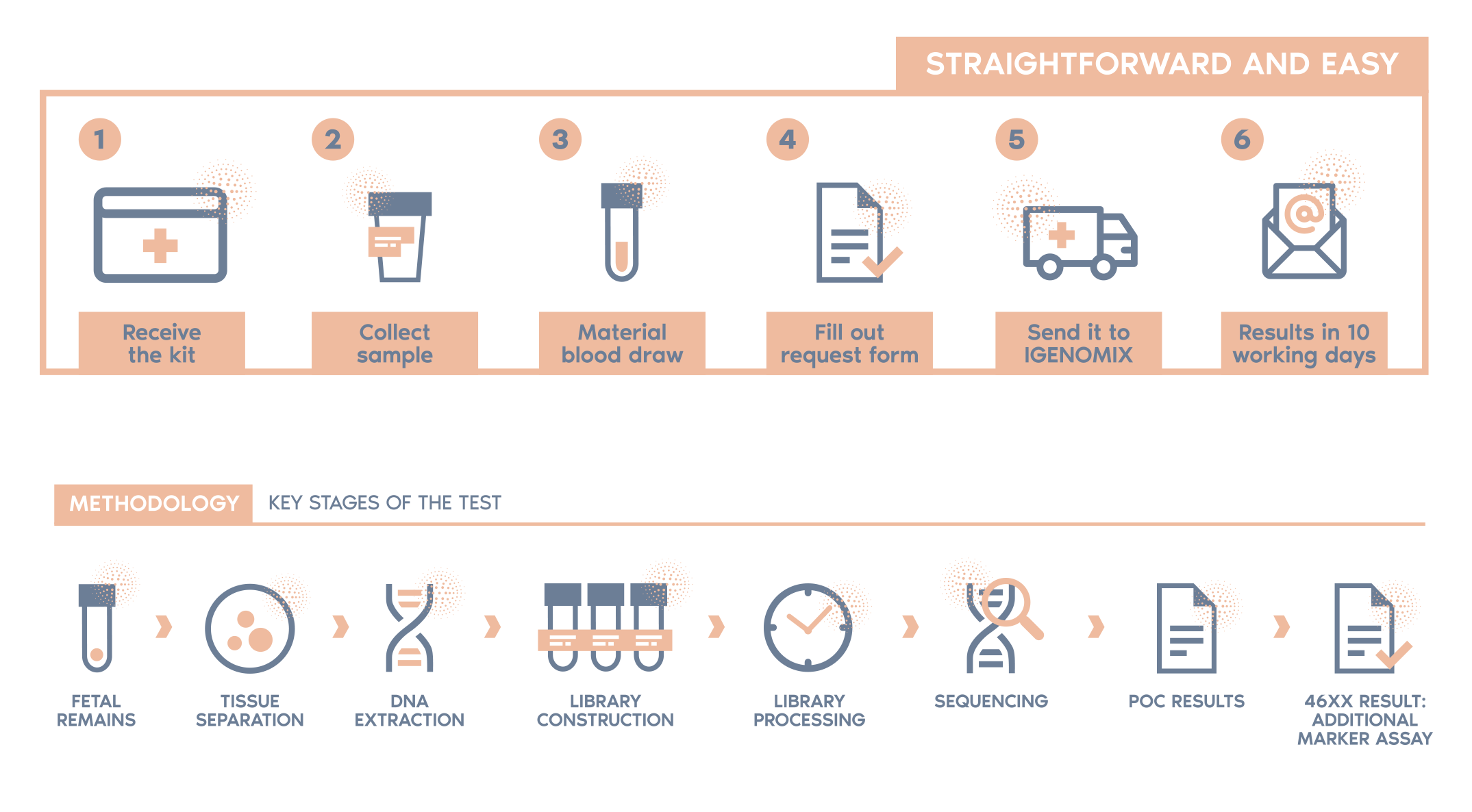
What is the procedure?